Study Guide
Field 240: Science: Chemistry
Sample Multiple-Choice Questions
Recommendation for individuals using a screenreader: please set your punctuation settings to "most."
Each multiple-choice question has four answer choices. Read each question and its answer choices carefully and choose the ONE best answer.
During the test you should try to answer all questions. Even if you are unsure of an answer, it is better to guess than not to answer a question at all. You will NOT be penalized for choosing an incorrect response.
The following reference material will be available to you during the test:
Periodic Table
Objective 0001
Understand practices of science and engineering.
1. Use the information below to answer the question that follows.
| Alcohol % | Water % | Measured Freezing Point (°C) |
|---|---|---|
| 10 | 90 | 25 |
| 20 | 80 | 20 |
| 30 | 70 | 5 |
| 40 | 60 | 0 |
| 50 | 50 | negative5 |
| 60 | 40 | negative10 |
| 70 | 30 | negative20 |
| 80 | 20 | negative35 |
| 90 | 10 | negative70 |
| 100 | 0 | negative130 |
A student designs a study to determine how adding isopropyl alcohol to water changes the freezing point of the solution. The table shows the freezing points measured by the student for each condition. The student uses this information to draw a number of conclusions. The teacher, however, notices that the student measured the freezing points in Fahrenheit and not in Celsius as labeled in the table. Which of the following conclusions by the student is most affected by the student's mislabeling of the temperature unit?
- Increasing the percentage of alcohol in a solution decreases the freezing point of water.
- The freezing point of pure water is different from the freezing point of pure isopropyl alcohol.
- A solution of 40 percent isopropyl alcohol and 60 percent water has the same freezing point as pure water.
- Changing the percentage of water in the solution results in an inversely linear change in the measured freezing point.
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: C.
The freezing point of pure water is 32 degreesF or 0 degreesC. When analyzing the table, a student might conclude that a solution of 40 percent isopropyl alcohol and 60 percent water has the same freezing point as pure water. This type of misconception is formed when data is mislabeled. In this case, the table is labeled in Celsius but the data were measured in Fahrenheit. Therefore, in reading the table, the student will infer that the 40/60 solution has a freezing point of 0 degreesC, when in actuality the freezing point is 0 degreesF, which is not the freezing point of pure water.
Objective 0002
Understand crosscutting concepts and their applications across science and engineering disciplines.
2. Which of the following sequences best describes the changes in energy forms as first a plant grows, then it is consumed by a rabbit, and finally the rabbit runs through a field?
- radiant energy becomes chemical energy becomes kinetic energy
- geothermal energy becomes radiant energy becomes chemical energy
- potential energy becomes mechanical energy becomes kinetic energy
- geothermal energy becomes potential energy becomes chemical energy
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: A.
The radiant energy from the sun is transferred into chemical energy during the process of photosynthesis when sunlight, water, and carbon dioxide are taken in by the plant to produce sugar and oxygen. The energy is stored as a sugar and can be either used for the function and/or growth of the plant. When the plant is consumed by the rabbit, the plant is digested as food and converted into chemical energy through metabolic processes. These chemical reactions produce and store energy in the form of macromolecules. As the rabbit begins to run, that stored chemical energy is converted into kinetic energy, enabling the rabbit to move and function.
Objective 0003
Understand the process of reading, and apply knowledge of strategies for promoting students' reading development in the science classroom.
3. Students in a chemistry class will be reading a text that focuses on the complex process of reaction mechanisms and transition states. The teacher wants to teach students how to use visualization as a strategy to improve their comprehension of the text. The teacher begins by explaining that visualization is used to create a mental picture of an object, concept, or process described in a text. Which of the following steps should the teacher take next in teaching this comprehension strategy?
- reading aloud a section of the text to students and prompting them to make a detailed drawing of the text's main idea
- using think-aloud to model the strategy while reading a section of the text aloud to students
- asking students to read the text and analyze how the author uses graphic features to support comprehension
- having students read the text silently and then point to a key illustration or graphic in the text
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: B.
A think-aloud process allows teachers to describe things they are doing to monitor comprehension while reading a text orally. By modeling how to use visualization as a comprehension strategy, the teacher shows students how skilled readers construct meaning from a text by generating mental images as they read. The students can then apply the teacher's visualization strategy to create their own images to help them understand the text they are reading.
Objective 0003
Understand the process of reading, and apply knowledge of strategies for promoting students' reading development in the science classroom.
4. An environmental science teacher would like to promote students' ability to use evidence from a text to support their writing and class discussions about assigned informational and expository texts. The teacher could best achieve these goals by providing the students with explicit modeling and practice in which of the following strategies?
- defining new vocabulary in their own words
- using visualization during reading
- identifying a text's organizational structure
- annotating a text during reading
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: D.
The process of annotating a text during reading enables students to engage in a close reading—a thoughtful, critical analysis of a text that focuses on significant details—to develop a deeper understanding of the text. When annotating a text, students highlight/underline key information and make notes of key ideas and questions. These notes help students formulate their thoughts for expression in writing and class discussions.
Objective 0004
Understand the disciplinary core ideas of chemistry.
5. Use the incomplete chemical equation below to answer the question that follows.
3 M G C L 2 + 2 F Ee yields 2 F E C L with an unknown subscript + 3 M G
In order to complete the equation shown, the subscript on chlorine on the product side of the equation must equal which of the following values?
- 1
- 2
- 3
- 4
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: C.
Chemical equations must follow the law of conservation of mass. Therefore, the number of atoms for each element on the reactant side is equal to the number of atoms for each element on the product side. In this example, magnesium chloride has a coefficient of three and chlorine has a subscript of two. Since there is no subscript on magnesium, the number of atoms is determined by what its coefficient is. In this case, there are three magnesium atoms on the reactant side of the equation. Similarly, there are two iron atoms on both the reactant and product sides. To determine the number of chlorine atoms, the coefficient number and the subscript number of that element are multiplied together, giving a total of six chlorine atoms in this compound. Thus, there needs to be six chlorine atoms on the product side of the equation. As the equation is presented, iron chloride has a coefficient of two, giving a total of two chlorine atoms. However, in order to balance this equation, there needs to be a total of six chlorine atoms. This is achieved by placing a subscript of three on the element chlorine. This will result in there being two molecules of iron chloride that each contain three atoms of chlorine. This equals a total of six chlorine atoms on the product side, which equals the reactant side and balances the chemical equation.
Objective 0005
Understand the disciplinary core ideas of physics.
6. Use the graph below to answer the question that follows.
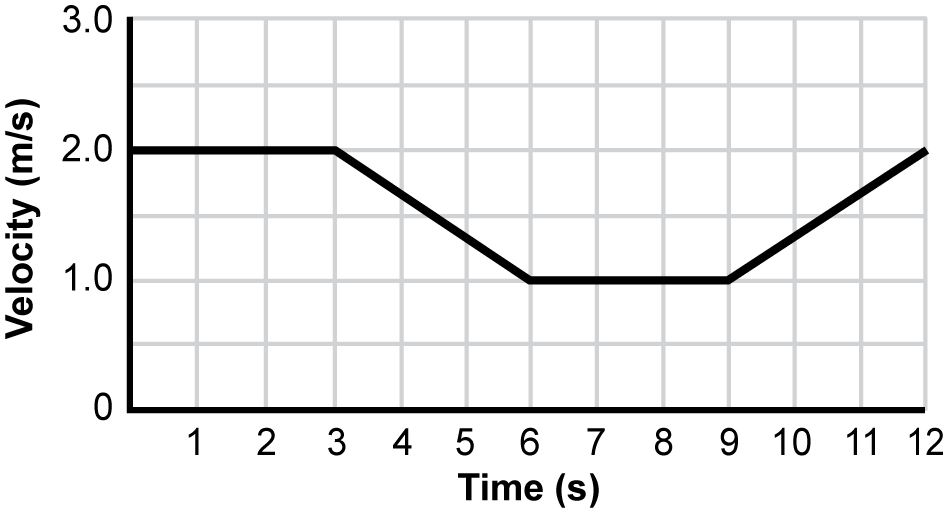
A graph of velocity in M per S versus time in S is shown. From 0 to 3 S, there is a flat line at 2 M per S. Then, there is a line with a negative slope from 3 to 6 S that goes from a velocity of 2 M per S to 1 M per S. Next, from 6 to 9 S, there is a flat line at 1 M per S. Finally, between 9 and 12 M per S, the line has a positive slope that goes from a velocity of 1 M per S to 2 M per S.
The graph shows the velocity of a person on a bicycle traveling on a straight, flat road. Within which of the following ranges is there a net force on the bicycle in a direction that is opposite to the velocity of the bicycle?
- 0to3 s
- 3to6 s
- 6to9 s
- 9to12 s
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: B.
On a velocity versus time graph, a negative slope, as shown between 3 and 6 seconds on the graph, indicates a decrease in velocity over time. The bicycle is slowing down because the net force is in the opposite direction of the velocity of the bicycle. This is due to the fact that the magnitude of the frictional force is greater than the magnitude of the applied force. However, the bicycle is still moving forward because velocity is the amount of displacement of the bicycle over a given time.
Objective 0005
Understand the disciplinary core ideas of physics.
7. A physical science teacher wants to relate motion and kinematics concepts to real-world events that occur in daily life. Which of the following student activities would best accomplish the teacher's goal?
- Students carry out an imaginary car ride and analyze changes in displacement and velocity that occur.
- Students measure the work done in pulling a box filled with books across a rough floor.
- Students analyze the forces acting on a piece of classroom furniture and construct a free-body diagram.
- Students visit a commercial power plant and describe the ways in which energy changes forms.
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: A.
The best activity to demonstrate motion and kinematics concepts in a classroom is to have students relate to these concepts by using real-world experiences. Students can use their real-world experience to perform an imaginary car ride and analyze what will happen to a car's displacement and velocity when it changes speeds, reverses, stops, or maintains a constant speed.
Objective 0006
Understand the disciplinary core ideas of biology.
8. Students use a molecular model kit with various colors of beads and are asked to create six carbon dioxide molecules and six water molecules. These bead molecules are then used to represent the process of photosynthesis. This bead model directly shows which of the following aspects of photosynthesis?
- The number of molecules of water produced in photosynthesis is equal to the number of reactant molecules of water.
- The oxygen that is produced as a result of the photosynthetic process is derived from both carbon dioxide and water.
- The splitting of water molecules into hydrogen and oxygen in photosynthesis is driven by the absorption of light energy.
- There is a conservation of matter during the reaction that produces one glucose molecule.
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: D.
The activity described directly shows the conservation of matter because students can observe the number of atoms per element before and after a chemical reaction. When students build the carbon and water molecules, they are able to observe and count how many atoms of carbon, hydrogen, and oxygen are present before the reaction occurs. Then, as the students represent the products of these reactions, they can observe that the atoms representing these elements were neither removed nor added but rearranged to form a glucose molecule during the reaction.
Objective 0007
Understand the disciplinary core ideas of Earth and space science.
9. Which of the following wind patterns is produced when warm air rises under high pressure and deflects right as Earth rotates about its axis?
- Doldrums
- Polar easterlies
- westerlies
- northeast trade winds
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: D.
The northeast trade winds, also known as prevailing winds, develop when the warm air at the equator rises under high pressure and Coriolis forces. As the air rises and flows toward the north, Earth is also rotating about its axis, which produces the Coriolis effect and deflects winds to the right. Around 30-degrees North latitude, the air begins to cool and descend, while still continuing to deflect to the right. The wind moves from a northeasterly direction to a southwesterly direction, and eventually returns back to the equator.
Objective 0007
Understand the disciplinary core ideas of Earth and space science.
10. Use the passage below to answer the question that follows.
When developing earthquake-resistant buildings, many newer designs follow a base-isolation model. This technology enables buildings to remain suspended above the foundation as an earthquake hits, allowing for the foundation to move and not the building. This design relies upon the use of bearings that contain a lead core wrapped in alternating layers of steel and rubber and move with the building and the foundation's movement.
Which of the following improvements would optimize this base-isolation design?
- adding shock absorbent materials to the building
- changing all metals in the building structure to titanium
- surrounding all of the building's bearings with concrete
- adding points along the building that can fold with movement
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: A.
The base-isolation model is used to prevent damage to a building during an earthquake by displacing and isolating the motion of the building to its foundation. The functioning of this type of design is dependent on the material and height of the building. Shock absorbent materials are added to the design to prevent and minimize further damage to the building in the event that the original design fails and the building would move. The shock absorbent materials function by absorbing the kinetic energy, instead of displacing that energy, in order to protect the building from damage.
Objective 0008
Understand the disciplinary core ideas of environmental science.
11. Compared with other forms of environmental regulation, policies that promote full-cost pricing, such as taxation of pollutants, have the primary advantage of:
- coordinating the efforts of many nations to maximize the global impact.
- having more transparent and visible benefits.
- relying on the power of incentives to encourage efficient responses.
- working in a more time-efficient manner.
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: C.
Full-cost pricing is a policy wherein the price of a product is adjusted to account for any external costs, such as the costs associated with pollution. The taxation of pollutants has the effect of raising the price paid by consumers for a polluting product, or reducing the revenues and profits that its producers receive, or both. Consumers and producers then respond to these price incentives by finding ways to change the quantity of the product that they buy or sell, which reduces the quantity of pollution in the most efficient possible way.
Objective 0008
Understand the disciplinary core ideas of environmental science.
12. Students are using the engineering design process to create a filtration system that removes microplastic beads from a local freshwater aquatic ecosystem in order to reduce the presence of these beads in top-level predators. Background research needed to develop this filtration system would most likely include obtaining information about the:
- production and use of plastics in various consumer products.
- movement of microplastics through the food chain.
- availability of alternative plastic-free consumer products.
- current sources of microplastic pollution in global ecosystems.
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: B.
To accomplish the goal of this activity, the students need to have an understanding of how matter moves through the local watershed, including through the aquatic ecosystem. Therefore, the students must research the movement of microplastics to determine where the microplastics are in the environment and in which organisms they are concentrated. This will allow them to discover the best design and place for the filter.
Objective 0009
Understand atomic structure and theory, trends and relationships in the periodic table, and the nature of bonds.
13. By the advent of modern quantum mechanics in 19 26 , experimental evidence supported a model of the electron as:
- a matter wave in a spherical cloud of probability.
- continuously traveling in a fixed, circular orbit.
- a point particle with a definite position and momentum.
- incapable of releasing energy in jumps between orbits.
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: A.
Quantum mechanics views matter as exhibiting wave-like behavior. A beam of electrons can be diffracted like light, and the DeBroglie hypothesis describes the wavelength of matter waves.
Objective 0009
Understand atomic structure and theory, trends and relationships in the periodic table, and the nature of bonds.
14. The shape of ammonia, N H 3 , is trigonal pyramidal. Which of the following combinations of pure atomic orbitals correctly describes the hybridization of the central atom in N H 3 ?
- S P
- S P 2
- S P 3
- S P 3 D
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: C.
Nitrogen has five electrons in its outer shell. These five electrons pair with one electron each for the three atoms of hydrogen. This leaves one pair of electrons that is not part of a bond. This electron configuration uses the s orbital, which can hold two electrons and three p orbitals, which also hold two electrons. The orbitals combine to form four S P 3 hybrid orbitals to contain the bonds and an unshared pair. This arrangement when spread out evenly gives the trigonal pyramidal shape to the ammonia molecule.
Objective 0010
Understand the structure and properties of substances.
15. An ice cube left out on a countertop is observed to melt significantly on the bottom face, but almost no melting is observed on the top and sides in contact with the air. Which aspect of the kinetic molecular theory best explains this observation?
- The temperature of the countertop is greater than the air, leading to more effective collisions.
- The density of the air is less than the countertop, and there are fewer collisions between particles.
- The surface area exposed to air is greater than that exposed to the countertop, leading to more cooling.
- The rate of thermal transfer to a solid is always less than the rate of transfer to a gas.
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: B.
The kinetic molecular theory states that collisions between particles are perfectly elastic for ideal gases. Real gases, however, transfer energy with collisions, and the countertop is much more dense than the air. This means there are many more collisions with the particles of the countertop, which causes the rate of melting to be greater.
Objective 0010
Understand the structure and properties of substances.
16. While graphing data from a phase change lab, two students notice the slopes of their graphs do not match for the region of heating liquid water. If they both used the same mass of water from the same source and the same size and type of beaker, which of the following is the best explanation for this observation?
- One of the samples must have a contaminant.
- The energy applied must be unequal for the two samples.
- Each sample was heated evenly throughout the process.
- The total heat input was equivalent for the two samples.
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: B.
The graph of a phase change procedure indicates the change in temperature on the y axis and the progress of time on the x axis. The slope of this graph will indicate the rate of temperature change. Some segments will have a slope of zero, indicating no change in temperature. If all other conditions are the same, the only possible explanation is the heat applied is different for the two samples.
Objective 0011
Understand the characteristics and properties of solutions.
17. Ethylene glycol is a common component in automobile coolant. When mixed with water it raises the boiling point of the resulting solution. Which of the following effects will the addition of ethylene glycol also produce?
- increasing the effectiveness of hydrogen bonding between water molecules
- raising the specific heat of water so it can absorb more energy
- reducing the molar solubility of other compounds in the coolant
- decreasing the freezing point of the resulting mixture
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: D.
Ethylene glycol, like other solutes, affects the boiling and freezing points when dissolved in a solvent. It will raise the boiling point and lower the freezing point. This is one of the colligative properties of solutions.
Objective 0012
Understand the properties of matter and the principles of conservation of matter, mass, and charge.
18. Use the balanced chemical equation below to answer the question that follows.
4 N H 3 gas + 6 N Oh gas yields 5 N 2 gas + 6 H 2 Oh gas
If seventy seven grams of nitric oxide formula n o are used as a reactant, approximately how many grams of nitrogen gas will be produced?
- 10. g
- 28 g
- 60. g
- 92 g
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: C.
This is a stoichiometry problem that assumes excess ammonia in the reaction. First, divide 77 grams by the formula mass of N Oh. This is the number of moles of N Oh that will react. Next, multiply by the mole ratio from the balanced equation. It is (5 divided by 6) for nitrogen produced by nitric oxide. Finally, multiply this value by the formula mass of elemental nitrogen to obtain the grams of nitrogen produced.
Objective 0012
Understand the properties of matter and the principles of conservation of matter, mass, and charge.
19. Use the balanced chemical equation below to answer the question that follows.
C O gas + 2 H 2 gas yields C H 3 O H gas
In a study of this reaction, a chemist reacts 156.0 g of C O with 28.00 g of H 2 and allows the mixture to react to completion. What is the maximum mass of C H 3 O H produced from this reaction?
- 178.5 g
- 222.1 g
- 356.8 g
- 444.3 g
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: A.
This is a stoichiometry problem to find the theoretical yield for the given equation. The yield is obtained by dividing the mass of each reactant by its formula mass, then multiplying by the mole ratio for the equation. The ratio is 1 to 1 for carbon monoxide and 2 to 1 for hydrogen. This gives the number of moles of methanol that could be formed for each of the reactants, assuming the other reactant is present in excess. Choose the reactant that would produce the least number of moles of product. This is the limiting reactant. Multiply the number of moles produced by the formula mass of methanol, and the result is the number of grams formed in the reaction.
Objective 0013
Understand energy, thermodynamics, and equilibrium in chemical systems.
20. The standard enthalpy change of formation of calcium nitrate is represented by which of the following equations?
- C A solid + 2 N O 3 liquid yields C A open parens N O 3 close parens 2 solid
- C A solid + 2 N O 3 gas yields C A open parens N O 3 close parens 2 solid
- C A solid + N 2 liquid + 3 O 2 liquid yields C A open parens N O 3 close parens 2 solid
- C A solid + N 2 gas + 3 O 2 gas yields C A open parens N O 3 close parens 2 solid
- Answer and Rationale. Enter to expand or collapse. Answer expanded
-
Correct Response: D.
Enthalpy of formation uses the chemical species in their elemental form. The state of matter is determined by what state the substance is in at 1 K P A and two hundred ninety eight K. For calcium nitrate, the calcium is solid at these conditions, and the nitrogen and oxygen are gases.
